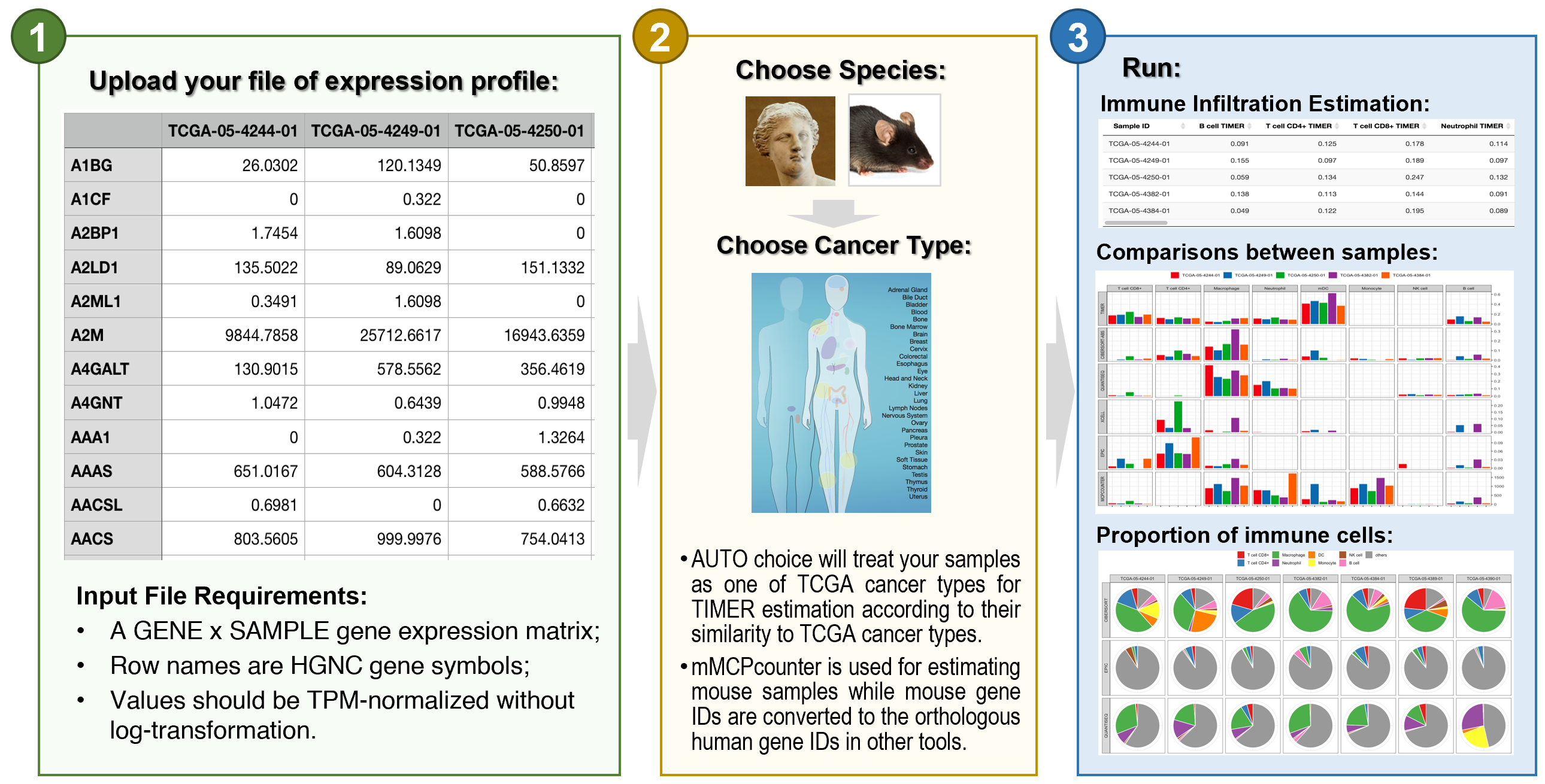
TIMER is a comprehensive resource for systematical analysis of immune infiltrates across diverse cancer types. This version of webserver provides immune infiltrates' abundances estimated by multiple immune deconvolution methods, and allows users to generate high-quality figures dynamically to explore tumor immunological, clinical and genomic features comprehensively.
Geting started by exploring:Gene_Outcome: association between gene expression and clinical outcome;
Gene_Mutation: differential gene expression between mutation status on gene;
Gene_Corr: correlation between genes.
Note: the estimation scores of TIMER2.0 might differ slightly from previous versions of TIMER due to the different number of available TCGA samples used for batch correction in the current run.
Tutorial:Cite us!
Taiwen Li#, Jingxin Fu#, Zexian Zeng, David Cohen, Jing Li, Qianming Chen, Bo Li, and X. Shirley Liu. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Research 2020 [DOI] [PubMed]
Taiwen Li, Jingyu Fan, Binbin Wang, Nicole Traugh, Qianming Chen, Jun S. Liu, Bo Li, X. Shirley Liu. TIMER: A web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Research. 2017;77(21):e108-e110. [DOI] [PubMed]
Bo Li, Eric Severson, Jean-Christophe Pignon, Haoquan Zhao, Taiwen Li, Jesse Novak, Peng Jiang, Hui Shen, Jon C. Aster, Scott Rodig, Sabina Signoretti, Jun S. Liu, X. Shirley Liu. Comprehensive analyses of tumor immunity: implications for cancer immunotherapy. Genome Biology. 2016;17(1):174. [DOI] [PubMed]
Taiwen Li:
litaiwen@scu.edu.cn
Jingxin Fu:
jxfu@ds.dfci.harvard.edu
X Shirley Liu:
xsliu@ds.dfci.harvard.edu
User Visiting Statistics
TIMER v2.0 | © X Shirley Liu Lab 2020 | Dana Farber Cancer Institute